School of Environmental Sciences
Priyadharshini Hills P. O
Athirampuzha
Kottayam, Kerala, India-686560
P +91 2732120, +91 9447 391 168
E cta@mgu.ac.in
F +91 481 271 009/02
Priyadharshini Hills P. O
Athirampuzha
Kottayam, Kerala, India-686560
P +91 2732120, +91 9447 391 168
E cta@mgu.ac.in
F +91 481 271 009/02
The International Conference on Membranes (ICM-2013)
on October 3rd to 6th2013
on October 3rd to 6th2013
International Conference on Frontiers of Mass Spectromerty (ICMS 2013)
on September 6th to 9th 2013
on September 6th to 9th 2013
Second International Conference on Advanced Oxidation Processes (AOP 2012)
on October 5th to 8th 2012
on October 5th to 8th 2012
MEMBRANE RESEARCH
Membrane science and technology and its applications in industry are an upcoming field of research. The easy, less expensive and efficient methods of membrane processes have a promising future in physical applications such as separation processes, energy conversion and biochemical applications such as drug delivery systems, bio sensors, bio reactors etc.
An absolute definition for membrane which describes all the characteristics of it is very difficult. In the most general sense, a membrane could be defined as a phase that act as a barrier to prevent mass movement but allows restricted and/or regulated passage of one or more species through it (Lakshiminarayanaiah, 1984). Membranes can also physically or chemically modify the permeating species or regulate the rate of permeation. Thus membrane may be either passive or reactive depending on the membrane’s ability to alter the chemical nature of the permeating species (Lloyd, 1985).
Membrane Classification
Membranes can be classified according to their structure, source material, phase, purpose etc. They may be broadly classified as natural or synthetic based on their source. The major materials used for the preparation of synthetic membranes are ceramics, polymer and glass. Metals and liquids are also used for preparing some membranes.
Based on their mechanism of action, membranes can be classified as adsorptive, diffusive, ion-exchange or osmotic membranes(R. E. Kesting, 1971).Furthermore, membranes come in two typical structural configurations: their structure can be microporous or asymmetric.
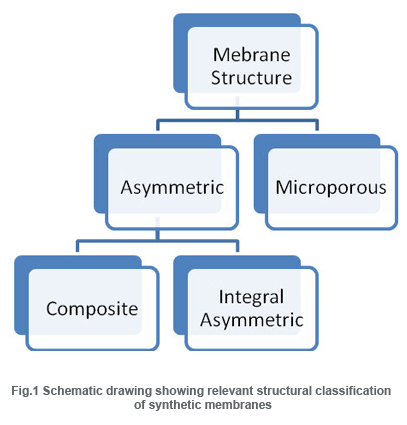
Microporousand Asymmetric membranes
Microporous membranes may have uniform pore size throughout the entire cross section (isotropic) or the pore size may vary along the cross section (anisotropic). Unlike microporous membranes, asymmetric membrane consists of a very “skin” layer on a highly porous substrate and varies in their structure and properties over their cross section. The thin skin on top of the asymmetric porous structure determines its separation characteristics. They are mainly used in pressure driven separation processes.The asymmetric membranes obtained from the phase inversion process are generally called “integrally skinned” membranes. Integral asymmetric membranes may consist of porous or homogeneous skin layer made up of ceramics, graphite, metal or metal oxides, and various polymers. If the skin layer in the asymmetric membrane is formed by deposition from solution or plasma, it is termed as “nonintegrally skinned” structure otherwise called composite membranes. Since the composite membranesare usedhere frequently, they are being discussed in the following sessions.
Composite membranes
Composite membranes have a thin dense homogeneous polymer skin formed over a microporous support film. While integrally skinned membranes are made in a one-step procedure, composite membranes are made in two or more steps.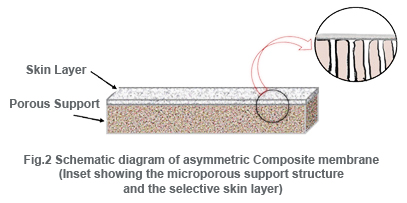
There are four general procedures for the preparation of composite membranes:Casting of the barrier layer separately and then laminating it on the microporous support film, Coating of the microporous support film by a polymer, Gas-phase deposition of the barrier layer on the microporous support film and Interfacial polymerization of reactive monomers on the surface of the microporous support film.
Another method fabrication of complex composite materials is Langmuir-Blodgett (LB) technique, in which monolayers are formed on a water surface followed by transferring in to a solid support. The LB technique was later prevailed by the layer-by-layer (LbL) technique.
LbL technique
The LbL technique was first mentioned by Iler in 1966, which was then expanded by Decher and Hong.This technique is a template assisted assembly in whichthin films are fabricated by depositing alternating layers of positive and negative charged materials with wash steps in between.The general methods of deposition involve solution dipping, spraying or spin coating. The formation of layers by spraying was introduced by Schlenoff and the use of spin coaters was demonstrated by Hong and also by Wang. Simple immersion of substrate (of suitable charge) in the solution of oppositely charged (required) material is the easiest way to form a bilayer. The main driving force of multilayer formation is electrostatic attraction between oppositely charged materials. The other interactions include donor/ acceptor interactions, hydrogen bonding, adsorption/drying cycles covalent bonds, stereocomplex formation or specific recognition.
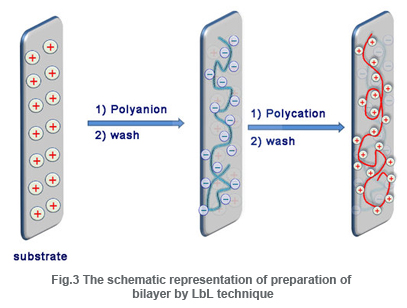
The materials used for coating can be small organic molecules or inorganic compound, macromolecules, biomacromolecules such as proteins or DNA or even colloids. The technique can be applied to any solvent accessible surfaces of various size and shapes.
The multimaterial assembly of several compounds using LbLtechnique can be employed to form multilayer films with complex functionality for surface interaction and surface based devices. Multilyers are successfully tailored for many needs like corrosion protection, antireflective coatings, antistatic coatings, surface induced nucleation, anti-fouling, hydrophilicity or hydrofobicity, biocompatibility, antibacterial properties, molecular recognition, molecular separation, chemical sensing or biosensing, microchannel flow control etc.Surface based devices like membrane reactor, photonic devices, and separation membranes are also being fabricated via LbL method.
The advantages of LbL technique are that it is simple, environmentally friendly and ultra-low coast. Another important desirable quality of LbL is the high degree of control over wide range of parameters like thickness, charge density, concentration, adsorption time, ionic strength and temperature. In some cases pH of the solution can be used as a parameter to control the properties of multilayers. The bilayers formed are so thin that this method offers controlled fabrication of nanocomposite multilayer.
Membrane Separation Processes
Various membrane separation processes like Reverse Osmosis (RO), Nanofiltration (NF), Ultrafiltration (UF), Microfiltration (MF), Dialysis, Electrodialysis and pervaporation are used for wide range of and applications and separation of particle/molecular sizes ranging from large molecules and suspended particles to ions. The pressure driven processes- RO, NF, UF, MF etc. vary in their transport propertiesmainly due to the variation in hydraulic pressure applied. Electrodialysis (ED) uses ion-exchange membranes to transport salt ions from one solution to another solution under the influence of an applied electric potential difference.Microfiltration is employed to remove suspended particlesin micron range (0.1mm-5 mm) like starch, pollen, latex etc. from dissolved substances by applying pressure below 20 psi. For the purification, concentration and fractionation of macro-molecules, Ultrafiltration is used which requires 20 - 70 psi pressure. Separation of ions, dissociated acids of the order 5- 10Aois made by Nanofiltration by applying 150 - 450 psi pressure. Reverse osmosis requires 500-1500 psi pressure and is able to eliminate monovalent salts and undissociated acids of size ranging from 1-5Ao. Intense research works are going on in membrane processesinwaste water treatment, dewatering and desalination to produce fresh water that is suitable for human consumption or irrigation.
Membranes for Energy Conversion
Membranes play a significant role in emerging low emission renewable energy technologies. Fuel cell is one of the most promising clean energy sources since it does not emit harmful substances. Among various kinds of fuel cells, polymer electrolyte membrane fuel cells can be easily engineered as energy source for automobiles, portable devicesas well as domestic applications. The most widely used solid polymer electrolyte membranes in fuel cells are perfluorosulfonic acid membranes such as Nafion, Acipex and Flemion. Different varieties of composite membranes containing inorganic nanoparticle fillers like TiO2, ZrO2, Al2O3 etc. has been prepared in fuel cells. But to make the techniques more applicable, the challenges like durability, cost reduction and water and air management has to be overcome. Composite membranes like Pd-alloy membranes supported on a porous metal are used in Hydrogen separation applications. Thermally stable high performance hydrogen separation membranes with high mechanical strength are being engineered recently.
Membrane Bio-Reactor(MBR)
Membrane Bio-reactor technology combines the biological degradation process by activated sludge with a contaminant separation by means of membrane separation processes. Instead of using gravitational settling or other separation methods, ultrafiltration or microfiltration techniques can be used for the retention of all the suspended impurities and microorganisms in the bioreactor. Thus MBR prevails over the conventional waste water treatment by having the advantages like small reactor requirements, high effluent quality, good disinfection, higher volumetric loading and less sludge production (Wisniewski, 2007).
Future of membrane technology
Membrane processes and applications are becoming a crucial technique in industrial processes, environmental conservation and energy production. Therefore fundamental research on membrane properties and transport phenomena in the various membrane operations is inevitable for the future of the membrane science and technology. Advanced membranes with high flux and high separability along with reduced concentration polarization and zero fouling for large scaleapplications will be the greatest concern in the recent future. The high energy efficiency, operational simplicity, ease of manufacture, transport selectivity and environmental compatibility makes membrane technology an important tool for advanced separation processes and chemical transformations by rectifying the limits encountered in the present scenario.
Our relevant Publications:
| 1. | M. S. Baburaj, C. T. Aravindakumar, S. Sreedhanya, A.P. Thomas and U. K. Aravind, Treatment of model effluents with PAA/CHI and PAA/PEI composite membranes, Desalination, 288 (2012) 72-79. |
| 2. | J. Mathew, S. Sreedhanya, M. S. Baburaj, C. T. Aravindakumar and Usha. K. Aravind, Fabrication of switchable protein resistant and adhesive multilayer membranes, Journal of Colloids and Interface Science, 94 (2012) 118-124. |
| 3. | J. Mathew, S. Sreedhanya, M.S. Baburaj, C.T. Aravindakumar and U.K. Aravind, Fabrication of switchable protein resistant and adhesive multilayer membranes, Colloids and Surfaces B: Biointerfaces 94 (2012) 118-124. |
| 4. | U. K. Aravind, B. George, M. S. Baburaj, S. Thomas, A. P. Thomas and C. T. Aravindakumar, Treatment of industrial effluent using polyelectrolyte membranes, Desalination, 27 (2010) 252-257. |
| 5. | U. K. Aravind, J. Mathew and C.T. Aravindakumar, Transport studies of BSA, lysozyme and ovalbumin through chitosan/polystyrene sulfonate multilayer membrane, Journal of Membrane Sciences, 299(2007), 146-155. |
| 6. | J. Mathew, C. T. Aravindakumar and U. K. Aravind, Effect of ionic strength and protein concentration on the transport of proteins through chitosan/polystyrene sulfonate multilayer membrane, Journal of Membrane Sciences, 325 (2008), 625- 632. |
References:
| 1. | M. Cheryan, Ultrafiltration and Microfiltration Handbook, Technomic Publishing Co., (1998). |
| 2. | H. Strathmann, Introduction to membrane science and technology, Wiley-VCH, (2000). |
| 3. | N. Lakshminarayanaiah, Equations of Membrane Biophysics, Academic Press, New York (1984). |
| 4. | R. E. Kesting, Synthetic polymeric membranes, McGraw-Hill book company, USA, (1971). |
| 5. | M.T. Ravanchi, A. Kargari, New advances in membrane technology, (2009). |
| 6. | K.B. Blodgett, I. Langmuir, Build-up Films of Barium Stearate and Their Optical Properties, Physical review, 51, (1937). |
| 7. | R.K. Iler, Multilayers of Colloidal Particles, Journal of Colloidal Interface Science, 21, (1966),569–594 |
| 8. | G. Decher, J.Hong, Buildup of Ultrathin Multilayer Films by a Self-Assembly process: III. Consecutive Adsorption of Anionic and Cationic polyelectrolytes on Charged Surfaces, Thin Solid Films,(1992). |
| 9. | G. Decher, J.B. Schlenoff, Multilayer thin films- Sequential assembly of Nanocomposite materials, Wiley-VCH, (2003). |
| 10. | Y.M Lvov, G. Decher, Assembly of Multilayer ordered films by alternating Adsorption of Oppositely charged Macromolecules, Crystallography Reports, (1994). |
| 11. | G. Decher, Science, 277, (1997), 1232. |
| 12. | B. Tieke, F. van Ackern, L. Krasemann and A. Toutianoush, Ultrathin self-assembled polyelectrolyte multilayer membranes, The European Physical Journal E, (2001), 29-39. |
| 13. | S.M. JavaidZaidi, T. Matsuura, Polymer Membranes for Fuel Cells, Springer, (2009). |
| 14. | C. Wisniewski, Membrane bioreactor for water reuse, Desalination, 203, (2007), 15-19. |
Home | Biography | Publications | Our Lab | News | Events | Erudite | SECAS | Research
Group | Collabrotions | Photos | Contact
Group | Collabrotions | Photos | Contact
| CTAMGU © 2012 | Design & Developed By : ITS Developers |


